
Introduction
The chemical industry is facing an increasing number of challenges. For example, the emergence of multidrug-resistant pathogens requires the development of new antibiotic drugs to protect vulnerable populations. Furthermore, with a constant increase in drug consumption in the aging population, the development of fabrication processes with lesser environmental impact is of the utmost importance. In this context, organic synthesis plays a vital role. Indeed, the development of new synthetic methods for the creation and evaluation of new molecules of biological interest is the foundation on which we improve the drugs available to treat illnesses. It can also serve to improve the manufacturing processes of known drugs and reduce their environmental footprint.
Our research program focuses on the development of new synthetic methodologies with the aim of improving the access to compounds with high-value to the industry. Our group has a unique expertise in both experimental and computational organic/organometallic chemistries. Consequently, we rely on joint experimental/computational studies to accelerate the research process and enhance our understanding of the studied reactions. Our group focuses on three main areas of research: 1) Iodine(III)-mediated Chemistry; 2) Organometallic Catalysis; 3) Computational Chemistry.
Iodine(III)-mediated Chemistry
Oxidative transformations are key synthetic methods as they enable rapid increase in molecular complexity. For this reason, tremendous efforts have been put forward to improve this class of useful processes. The development of hypervalent iodine reagents and their application in synthesis have been of particular interest in this regard. They tend to be eco-friendly, being carbon-based and usually biodegradable, and have been found to be in numerous cases a good alternative to toxic heavy metal-based reagents. In particular, iodine(III) reagents have served in a diverse array of synthetic methodologies, such as phenolic dearomatization chemistry, which has enabled access to certain complex molecular structures much more rapidly.
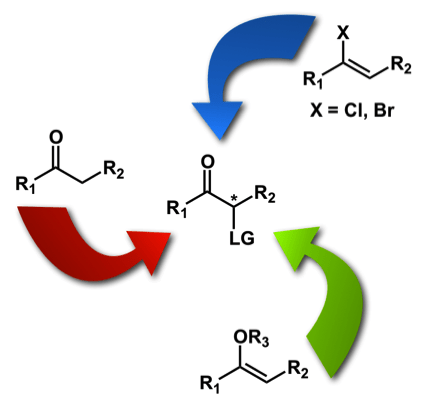
Axis 1 – Development of oxidative methodologies on ketones and enol surrogates: We have worked since 2009 on the development of iodine(III)-mediated reactions to access chiral nonracemic α-substituted ketones. The development of practical methods to access these chiral building blocks is of particular importance due to the ubiquitous nature of these products. We have investigated numerous strategies in this area: the direct α-tosyloxylation of ketones, the oxidation of enol esters, as well as the oxidative hydrolysis of haloalkenes. We have recently also developed an oxidative contraction of dihydropyranone derivatives to access useful substituted butyrolactones.
Axis 2 – Development of new iodine(III) reagents: A crucial part of the development of new iodine(III)-mediated reactions is the investigation of new types of hypervalent iodine compounds. In 2012, during the investigation of iodine(III)-mediated halogenation reactions, we reported the synthesis of a new fluoroiodane, which has been used by numerous groups in the past 6 years. More recently, we have investigated the properties and synthetic applications of a new family of iodonium zwitterions (see X-Ray structure)
Org. Lett. 2017, 19, 6420-6423
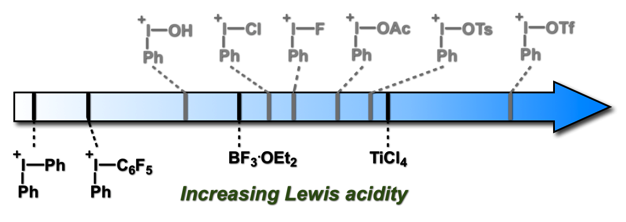
Axis 3 – Investigation of the properties and mechanisms of iodine(III) reagents: The development of better methodologies comes from a better understanding of the reagents involved in the processes. Using our unique expertise in computational chemistry, we have been investigating different iodine(III)-mediated reactions in the past years, such as the metathetical redox reaction of (diacetoxyiodo)arenes and Iodoarenes, and the α-tosyloxylation of ketones. We also have been working on the quantification of the Lewis acidity of iodonium species. This is a subject of particular interest, as their properties as Lewis acids is still not well known and could lead to novel catalysts with unique activities. Recently, we have greatly extended the quantification and understanding of the Lewis acidity of these compounds through a collaboration with the Mayr/Ofial group. We have demonstrated a novel application of this Lewis acidity with the Huber and Kirsch groups, with the Iodine(III)-mediated activation of organometallic catalysts.
Organometallic Catalysis
This field has revolutionized synthetic chemistry in the last 50 years. The diversity of accessible chemical transformations with organometallic catalysts makes it a tool of choice. To create an efficient catalyst, a balance between its stability and reactivity must be attained. These properties are controlled not only by the transition metal used, but also by the ligands on the complex. The synthesis of new types of ligands thus enable the creation of catalysts having new properties. We are particularly interested in the development of anionic carbene (NHC) ligands. Initially reported during my Ph.D. studies, my group is developing new families based on N-iminoimidazolium ylides. We have investigated and reported in 2013 an extended variant based on the acyl anionic tether. In 2019, we have reported a new ligand family based on a sulfonyl anionic tether. We have now reported a first catalytic application using novel Ir(III) catalysts based on these anionic NHC ligands. These catalysts, such as the one illustrated, are proficient to promote the coupling alcohols and anilines by Borrowing Hydrogen Catalysis (BHC).
Organometallics 2021, 40, 408-417
Computational Chemistry
A key tool in our research program is computational chemistry. It is through the theoretical study of reaction mechanisms that synergy arises between the experimental and computational fields. Since 2010, we have also shared our expertise with numerous groups:
- (4+1)-cycloaddition reaction of electron-rich carbenes – Claude Spino (UdeS, Canada)
- SN2 reaction of organofluorides – Jean-François Paquin (Université Laval, Canada)
- Origins of reactivity in Copper-catalyzed O-arylation of aminoalcohols – Alexandre Gagnon (UQAM, Canada)
- Origins of Chemoselectivity in NHC-catalyzed cross-benzoin reactions – Michel Gravel (University of Saskatchewan, Canada)
- Origin of selectivity in the synthesis of fluorinated iminosugars – Olivier Martin (Université d’Orléans, France)
- Selectivity of the Minisci reaction on cyclopropanol derivativeds – Arturo Orellana (York University, Canada)
- Denitrogenative Hydrotrifluoromethylation of Benzaldehyde Hydrazones – Graham Murphy (Waterloo University, Canada)
- Investigation of the properties of polyhalodicetylene – Pierre Baillargeon (CEGEP Sherbrooke, Canada)
- Pd-Catalyzed allylation of 4-alkylpyridines – Arturo Orellana (York University, Canada)
- Iodine-catalyzed synthesis of substituted furans and pyrans – Thanh Vinh Nguyen (UNSW, Australia)
- Pd-Catalyzed asymmetric borylative migration of enol perfluorosulfonates – Dennis G. Hall (University of Alberta, Canada)
Software Development
CYLview The efficient analysis and communication of scientific results are a vital portion of the work of any chemist. This aspect is even more important for computational chemists, due to the amount and complexity of computational results. The present major challenge with the preparation of publication quality representations of structures is the need to use multiple programs to achieve the desired result. In this regard, I created CYLview to accelerate the evaluation and analysis of computed structures, as well as to generate in the same program high quality representations, containing all the information (e.g. bond distances, angles, atom numbering) needed for professional publications and presentations. It is currently used by over 5000 computational chemists around the world and was cited in more than 1000 publications since 2009. A first release of the new version, CYLview20, has been online since October 2020.
CalcUS Computational chemistry is an increasingly active field due to the improvement of computing resources and theoretical tools. However, its use remains usually limited to technically-inclined users due to the technical challenges of preparing, launching and analyzing calculations. In this context, we have developed CalcUS, an open-source platform to streamline computational chem- istry studies. Its objective is to democratize access to computational chemistry by providing a user-friendly web interface to simplify running and analyzing quantum mechanical calculations. It is freely available, expandable and customizable. It promotes connectivity to multiple software packages and algorithms, thus providing state-of-the-art techniques to all practitioners. We propose CalcUS as a standalone tool and infrastructure to support other open-source packages.